Immunotherapies targeting immune checkpoint molecules PD-1/PD-L1 and CTLA-4 have revolutionised the treatment of cancer including melanoma1,2. These therapies unleash suppressed T cell responses allowing for reactivation of anti-tumour immune responses. Despite these advances, lack of response and severe immune related adverse events (irAEs) in some individuals, remain significant challenges for the field.
In recent years, the gut microbiome has gained much interest as a factor modulating anti-tumour immunity. Key clinical studies in melanoma and other cancers have identified associations between the microbiome and the efficacy of immune checkpoint inhibitor immunotherapies as well as the development of irAEs3-9. Phase I faecal microbiota transplantation trials in refractory melanoma patients have shown promising results, providing preliminary evidence to suggest that the microbiome can be targeted to overcome resistance to treatment in a subset of patients10,11. However, the “ideal” microbiome to facilitate immunotherapy response remains to be defined, with specific taxa linking the response and/or the development of toxicity across all cohorts lack consensus. In addition, dietary and geographic influences have not been well-studied.
We prospectively profiled the gut microbiomes and dietary patterns of 103 Australian and Dutch stage III melanoma patients treated with neoadjuvant anti-PD1/anti-CTLA-4 immunotherapy on trial at the Melanoma Institute Australia and the Netherlands Cancer Institute. We then performed an integrated analysis with data from 115 US melanoma patients.
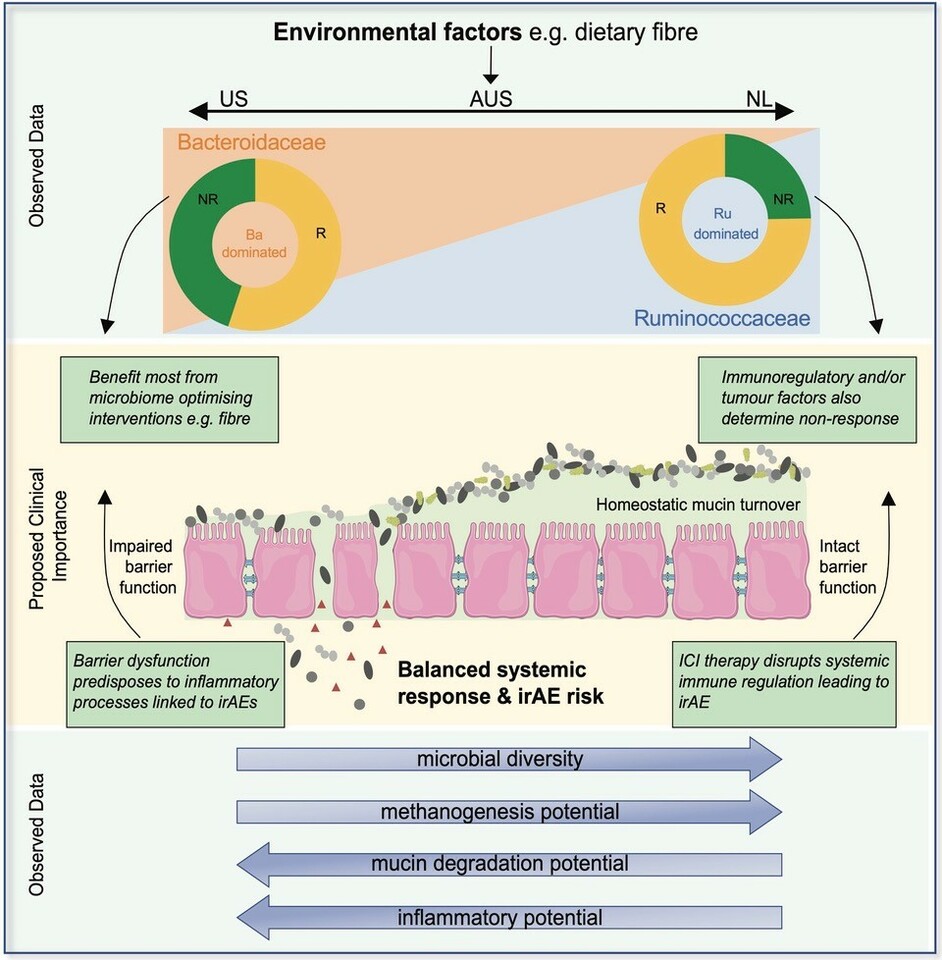
Through latent class analysis, identified differing microbial community assemblages in part linked to long term dietary patterns. On this basis, we classified patient microbiomes based on the ratio of Ruminococcaceae to Bacteroidaceae relative abundance. Stratifying patients in this way, there was a marked difference in the distribution of these microbial community structures between countries, which likely contributes to the lack of consensus in microbial biomarkers identified between cohorts from different centres and across countries. Of note, was the near absence of high diversity Ruminococcacae-dominated gut microbiomes in the US cohort and the near absence of the low-diversity Bacteroidaceae-dominated gut microbiomes in the Dutch cohort.
Notably, higher rates of response to immune checkpoint inhibitor immunotherapy were associated with Ruminococcaceae-dominated microbiomes, which had significantly higher alpha-diversity and were associated with higher fibre intake. In contrast, Bacteroidaceae-dominated microbiomes were associated with lower response rates, greater potential for microbial degradation of intestinal mucin and elevated inflammatory marker C-reactive protein in the peripheral circulation at baseline. Together, this cross-continental analysis highlights that differences in underlying gut microbial community ecology may underpin the relationship between gut microbes and clinical outcomes.
Our findings are highly relevant to the translation of microbial findings into the clinic, particularly dietary interventions. Not everyone responds in the same way to dietary interventions, and this is known to be dependent on the gut microbiome12,13. Accounting for differences in the assemblage of microbial communities will therefore be important to reproducibly targeting and altering the microbiome to improve immunotherapy outcomes. We hypothesise that increasing fibre in patients with Bacteroidaceae-dominated microbiomes, such as those from Australia and the US, will likely be of more clinical benefit in terms of improving the efficacy of immunotherapy than in patients who already have fibre imprinted microbiomes, such as those within our Dutch cohort. It also highlights that the gut microbiome is only one of many factors that influences the efficacy of treatment and is not the underlying driver of all immunotherapy resistance. Some patients irrespective of having high diversity and Ruminococcaceae-dominated microbiomes still did not respond to treatment. Modifying the microbiome will therefore likely only be effective at improving response in a subset of patients.
In support of recent studies14,15, our study further highlights the complexity of the relationship between the gut microbiome and clinical outcomes and that no single microbe alone is a fully predictive biomarker. However, we further demonstrate that microbial community ecology underpins both treatment outcomes and the role and predictive power of various microbes. Overall, our data provides novel insights into the relevance of diet and the gut microbiome in shaping response and toxicity to immunotherapy and demonstrates a path to optimising the utility of the microbiome in the clinic and the development of more targeted interventions to reshape the microbiome and improve treatment outcomes.
References
1 Wolchok, J. D. et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol 40, 127-137, doi:10.1200/JCO.21.02229 (2022).
2 Larkin, J. et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381, 1535-1546, doi:10.1056/NEJMoa1910836 (2019).
3 Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104-108, doi:10.1126/science.aao3290 (2018).
4 Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97-103, doi:10.1126/science.aan4236 (2018).
5 Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91-97, doi:10.1126/science.aan3706 (2018).
6 Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632-1640, doi:10.1126/science.aaz7015 (2021).
7 Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med 27, 1432-1441, doi:10.1038/s41591-021-01406-6 (2021).
8 Chaput, N. et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 28, 1368-1379, doi:10.1093/annonc/mdx108 (2017).
9 Frankel, A. E. et al. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 19, 848-855, doi:10.1016/j.neo.2017.08.004 (2017).
10 Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science, doi:10.1126/science.abb5920 (2020).
11 Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595-602, doi:10.1126/science.abf3363 (2021).
12 Kovatcheva-Datchary, P. et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab 22, 971-982, doi:10.1016/j.cmet.2015.10.001 (2015).
13 Zeevi, D. et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 163, 1079-1094, doi:10.1016/j.cell.2015.11.001 (2015).
14 McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med, doi:10.1038/s41591-022-01698-2 (2022).
15 Lee, K. A. et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med, doi:10.1038/s41591-022-01695-5 (2022).






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in