Treatment of non-small cell lung cancer patients without targetable oncogenic mutations and without PD-L1 expression remains a major challenge with an unmet medical need for new, rational therapeutic strategies1.
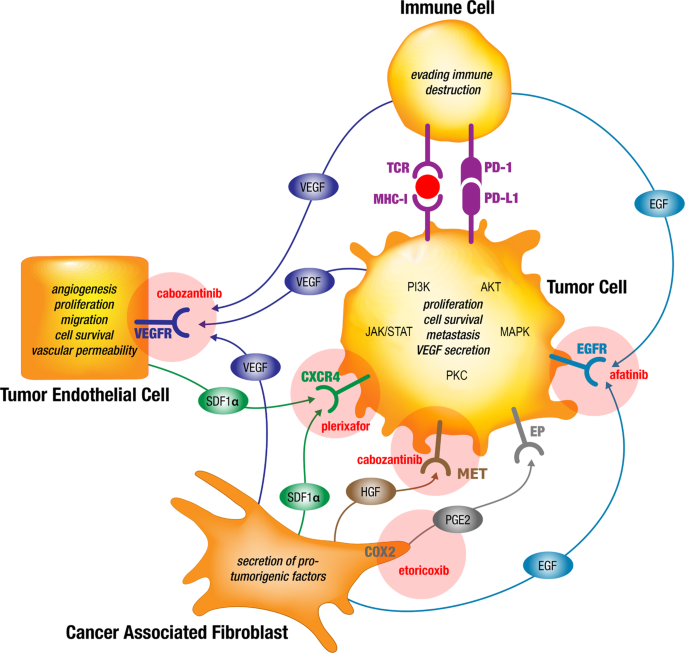
Tumors can be conceived as organs constituted of different cell types with distinct biologic functions, building a Cellular Tumorigenic Network with integrated inter- and intracellular signaling. Next to the neoplastic cells, originating from critical genetic alterations, non-neoplastic cells such as cancer associated fibroblasts, endothelial cells and immune cells comprise a heterogenic tumor microenvironment. Tumor cells are able to reprograms those associated cell types inducing processes such as neoangiogenesis, inhibition of apoptosis, immune suppression and induction of hypoxia and senescence. During the evolving tumor development and as response to targeted therapies, the Cellular Tumorigenic Network is reshaped further leading to heterogeneity and adaptive drug resistance in many cases2,3,4,5. This is partly based on substitutions for inhibited pathways and on crosstalk of generic intracellularly connected downstream pathways such as the mitogen-activated protein kinase (MAPK), Janus kinase and the signal transducer and activator of transcription protein (JAK/STAT), phosphoinositide 3-kinase (PI3K), protein kinase B (AKT) and protein kinase C (PKC)6.
The simultaneous and distinct inhibition of signaling within the Cellular Tumorigenic Network and suppression of cellular interdependency by targeting critical paracrine signaling axes is intended to inhibit tumor cell proliferation and thus to overcome drug resistances7.
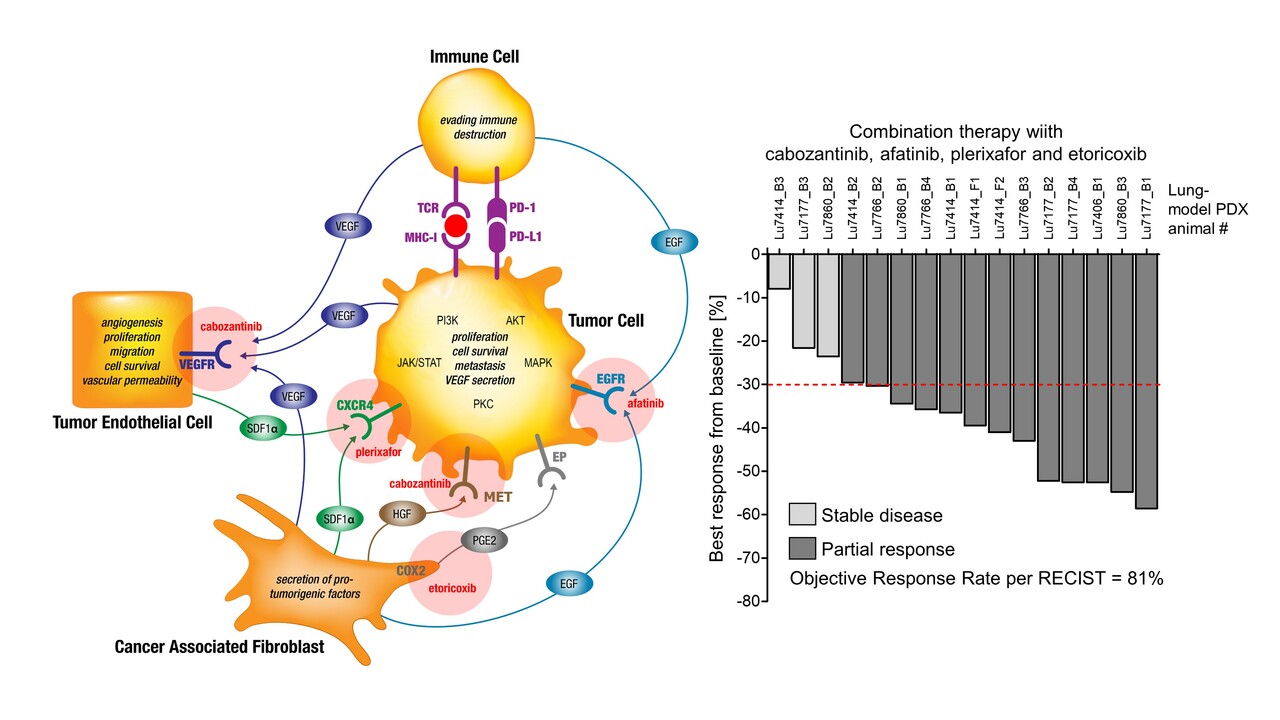
Based on this hypothesis we compiled a low-dose targeted drug regimen combining drugs inhibiting tumor-, endothelial-, immune- and cancer associated fibroblast cell interaction by blocking VEGFR and HGFR (cabozantinib), CXCR4 (plerixafor), EGFR (afatinib) and COX2 (etoricoxib). This parallel inhibition of paracrine signaling should effectively inhibit the generic intracellularly connected downstream MAPK, JAK/STAT, PI3K, AKT and PKC pathways.
For experimental validation of this hypothesis, five highly resistant patient derived non-small cell lung cancer xenograft models have been selected from a panel of more than thirty available tumors and subjected to treatment with the proposed drug combination regimen. All sixteen patient-derived lung cancers, including highly therapy-resistant adeno- and squamous cell carcinomas without targetable oncogenic mutations, were completely growth suppressed by this drug regimen, leading to an Objective Response Rate of 81% and a Clinical Benefit Rate of 100% with an excellent safety profile. Bioinformatics analysis of gene expression profiles for these lung cancer models provides evidence of a relationship between the expression of the paracrine signaling pathway members and common drug resistances which could be overcome by the new drug combination.
These results strongly encourage the further validation of this cabozantinib, afatinib, plerixafor and etoricoxib combination therapy in preclinical and clinical studies for advanced stage lung cancer patients without current therapeutic options.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 553, 446-454 (2018).
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18, 884-901 (2010).
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov 12, 31-46 (2022).
- Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 211, 1503-1523 (2014).
- Hahn WC, Bader JS, Braun TP, et al. An expanded universe of cancer targets. Cell 184, 1142-1155 (2021).
- Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med 5, (2015).
- Langhammer S, Scheerer J. Breaking the crosstalk of the cellular tumorigenic network: Hypothesis for addressing resistances to targeted therapies in advanced NSCLC. Oncotarget 8, 43555-43570 (2017).





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in