Most people are familiar with proteins, but few are well-acquainted with the sugar structures decorating them – the saccharides (“glycans”, among friends) that are attached to >80 % of proteins before surface expression or secretion.
That’s a shame, because glycans are immensely interesting. Especially because most FDA-approved cancer biomarkers are either glycans or glycoproteins, so they play a crucial role in clinical practice.(1) In addition, an intriguing fact is that we - since the 1960s - have known that a distinct class of glycans (“O-glycans”, which normally forms beautiful elaborated structures of oligosaccharides by on the cell surface) are shortened to a primitive state consisting of only one or two carbohydrate residues in cancers.
These short truncated, cancer-associated O-glycans have been known for ages, but still we have not yet managed to target them efficiently for the benefit of patients. Trials in 90’s and 00’s tried to use truncated O-glycans as targets for anti-cancer vaccines but failed to show effects – possibly due to the fact that patients were not stratified on whether their tumours actually expressed these epitopes.(2,3) However, with the advent of immunotherapies such as CAR-T cells and other T-cell redirecting therapies, the field has been reinvigorated. Several pre-clinical and clinical studies are under way trying to target O-glycans and the cancer-associated proteins they reside on to create hyper-specific, anti-cancer therapies.
A seminal paper in this endeavor showed remarkable effectivity of CAR-T cells targeting cancer-associated, truncated O-glycans in solid tumours in murine models.(4) Since then, CAR-T cells attacking these sugary sweet spots of cancer cells have been entering clinical trials and preliminary phase I data show a good safety profile, although results on efficacy are still not out.(5)
Several laboratories have spent time trying to understand why O-glycans become shortened on cancer cells. Most results point to the importance of changes in pH and the inflammatory environment and, in a few cases, genetic mutations affecting the enzymes essential for the elongation step in O-glycan biosynthesis. From a translational perspective, it is perhaps even more important that the short O-glycans activates cellular growth and stimulates a regulatory immune response,(6–8) suggesting that the expression of short O-glycans are tightly linked to the fitness of the cancer and making them an obvious target for immunotherapies.
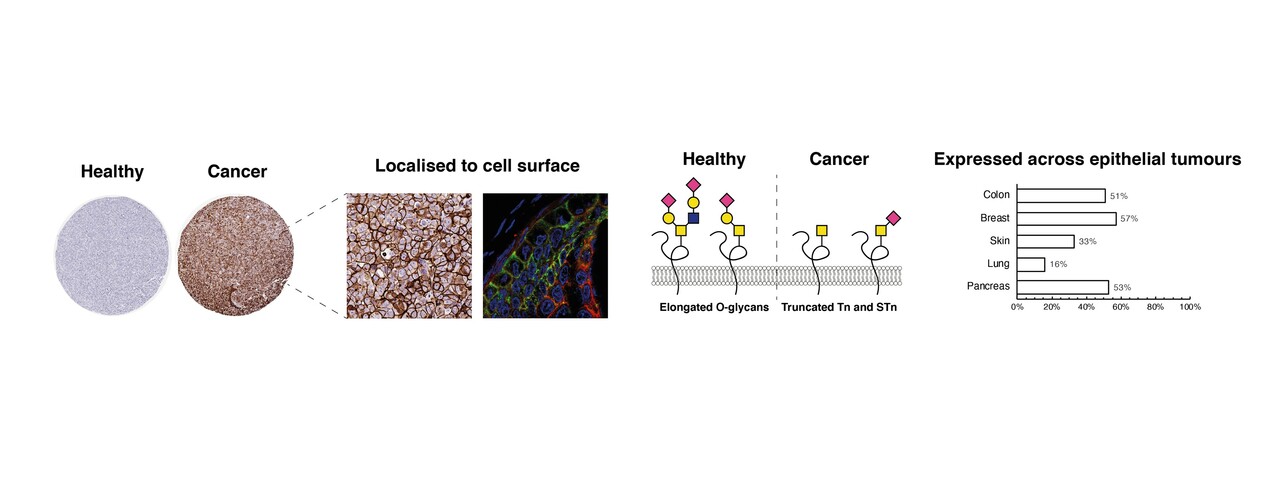
Working in the field for some years now, we noticed that most papers examining in which cancers these truncated O-glycans are expressed are from the 60s, 70s and 80s. This is naturally not a problem in itself, but still we found several issues with these older studies that needed to be addressed. The first is that most of these papers were based on detection techniques that often made it hard to distinguish the cancer-associated, truncated O-glycans from similar structures that just represent normal blood group antigens. The second is that most papers didn’t really distinguish between structure on the surface of the cells and those expressed as biosynthetic intermediates inside both cancerous and healthy cells. And the third is that many cancer types – and many healthy tissues, too - had not been properly, systematically surveyed for expression of truncated O-glycans using modern strategies.
In our paper, we use well-characterized, monoclonal antibodies against three different antigens (called Tn, STn and T) to stain over 700 tumour cores and show that these truncated O-glycans are indeed highly expressed on the surface of most tumours. Especially tumours from breast, pancreas and colon carried a lot of Tn and STn (50-80%). However, it was rarely 100 % of the tumour that stained positive for the antigens – but quite often (10-15% of the cases), it was a significant amount of the tumor (>70%) that carried these truncated sugars on their surface. Also, this expression is highly restricted to tumors from epithelial tissue. Tumours from the central nervous system turned out to only express the Tn-antigen on the surface in very few cases. The same was the case for tumours of mesenchymal lineage, where we in the few analyzed samples saw no surface reactivity. On the other hand, an important, and clinically relevant finding, was that healthy tissue showed in fact no surface reactivity for Tn – and only very little reactivity when it came to the STn-antigen.
Furthermore, tumours in earlier stage of dedifferentiation seems to show more Tn and STn-positivity. This align well with the fact that truncated O-glycans are often found in pre-malignant lesions, suggesting that these short sugars are onco-fetal structures and hallmarks of early cancer development. In addition, we found that tumours that had xenografted from patients and unto murine models also carried O-glycans in some cases. This could indicate that even some metastatic cancers carried these short sugars and could therefore be targeted.
So where does this leave us? With an up-to-date mapping of these drug-able and elusive structure based on modern day, straightforward immunohistochemical techniques. We have shown that these sugar structures are indeed highly expressed on the surface of many epithelial cancers – especially in breast, colorectal and pancreas cancers. And – importantly – they are seldom found in healthy tissue.
Hopefully this can pave the way for new, additional trials and experimental treatments seeking to help patients suffering from hard-to-treat, solid tumours – and hopefully, we can use this knowledge about cancer-associated glycans to make treatments that target both the glycan and the protein to which they are attached, making hyper-specific cancer treatments. That will require more work and innovation – but now, we have a map that can guide our travels.
- JA L, JN W. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer [Internet]. 2005 Nov [cited 2021 Sep 14];5(11):845–56. Available from: https://pubmed.ncbi.nlm.nih.gov/16239904/
- D M, H R, M M, TJ P, DA C, J G, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist [Internet]. 2011 Aug [cited 2021 Sep 14];16(8):1092–100. Available from: https://pubmed.ncbi.nlm.nih.gov/21572124/
- DW M, KE T, R G, M R, BM L, J T-P, et al. A randomised phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br J Cancer [Internet]. 1996 [cited 2021 Sep 14];74(8):1292–6. Available from: https://pubmed.ncbi.nlm.nih.gov/8883420/
- AD P, RD S, AC B, C S, U M, B E, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity [Internet]. 2016 Jun 21 [cited 2021 Sep 12];44(6):1444–54. Available from: https://pubmed.ncbi.nlm.nih.gov/27332733/
- Gutierrez R, Shah PD, Hamid O, Garfall AL, Posey A, Bishop MR, et al. Phase I experience with first in class TnMUC1 targeted chimeric antigen receptor T-cells in patients with advanced TnMUC1 positive solid tumors. https://doi.org/101200/JCO20213915_suppl.e14513. 2021 May 28;39(15_suppl):e14513–e14513.
- P R, S D, FB M, C F, KL K, C S, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci U S A [Internet]. 2014 Sep 30 [cited 2021 Sep 15];111(39):E4066–75. Available from: https://pubmed.ncbi.nlm.nih.gov/25118277/
- CBK M, MC C, S B, SR M, M I, P TS, et al. Genetically engineered cell factories produce glycoengineered vaccines that target antigen-presenting cells and reduce antigen-specific T-cell reactivity. J Allergy Clin Immunol [Internet]. 2018 Dec 1 [cited 2021 Sep 15];142(6):1983–7. Available from: https://pubmed.ncbi.nlm.nih.gov/30125661/
- Cornelissen LAM, Blanas A, Zaal A, van der Horst JC, Kruijssen LJW, O’Toole T, et al. Tn Antigen Expression Contributes to an Immune Suppressive Microenvironment and Drives Tumor Growth in Colorectal Cancer. Front Oncol. 2020 Aug 18;0:1622.







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in