Thousands of years of evolution have equipped us with sophisticated mechanisms of defense to ensure that foreign pathogens and malfunctioning cells are effectively eliminated. So, how can cancer cells overcome these intrinsic protectors? Moreover, multiple studies have shown that aggressive cancers do not only suppress the immune system but indeed turn these immune cells into cancer allies that ultimately favor tumor development. How to revert these features, and sensitize tumors to novel immunotherapies is the main challenge in oncology, in particular, in highly aggressive cases such as malignant melanoma.
Cutaneous melanoma, the most lethal type of skin cancer, is challenging because of its remarkable potential to metastasize even from very thin lesions. This process initiates primarily via the lymphatic system1. Nevertheless, the mechanisms that favor melanoma metastasis are unclear. Our group at the CNIO is particularly interested in this question. We started by live imaging, using unique mouse models we coined as “MetAlert” that had been engineered to visualize neolymphangiogenesis at the whole-body level. Using MetAlert, we identified the growth factor MIDKINE (MDK) as a new driver of (pre)metastatic niches in melanoma. The functional relevance of these findings was then demonstrated by finding MDK as an indicator of bad prognosis in melanoma patients. This study, which was published in Nature in 2017 (Ref2), set the basis for this current publication in Nature Medicine3.
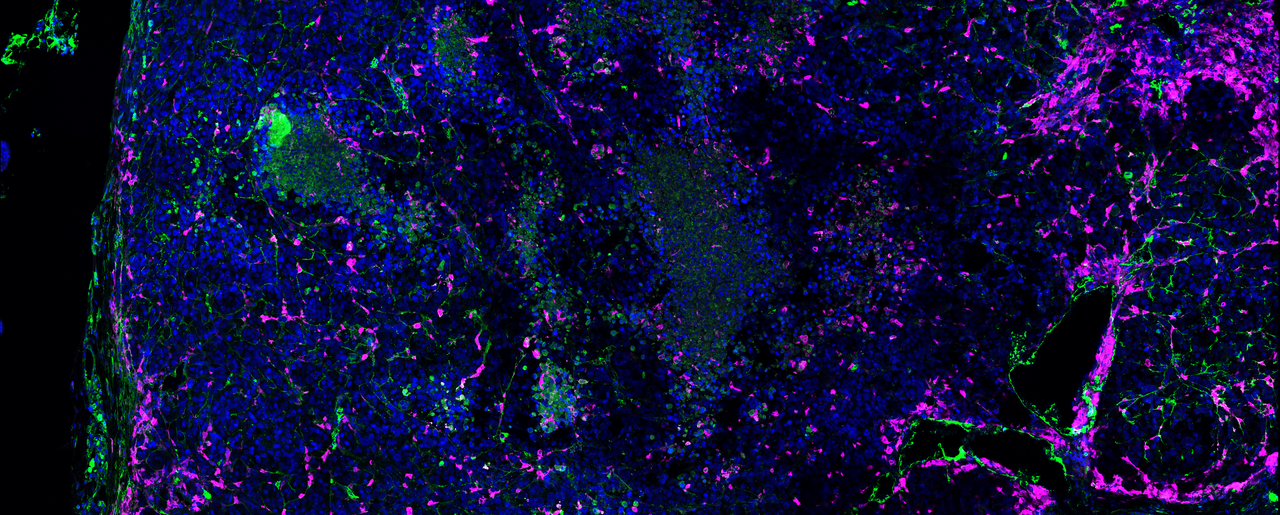
During further functional and histological characterization of MDK in mice, we had another striking observation. By immunofluorescence stainings of primary tumors, we realized that those tumors expressing MDK were depicting a remarkable infiltration of myeloid immune cells. This was curious as myeloid cells, particularly macrophages, are classical mediators of antitumoral immune responses. However, in the context of high-MDK tumors, myeloid cells showed classical immunosuppressive and pro-tumoral features, such as Arginase-1 expression. We then questioned whether, beyond roles on melanoma metastasis, MIDKINE could have further implications in immune evasion, and consequently, on immune therapy.
Histopathological studies of patient biopsies also showed an enrichment of tumor-associated macrophages in cases with high MDK levels. We then dissected the mode of action of MDK, finding that this protein exerts autocrine and paracrine effects in melanoma cells, ultimately resulting in an immune suppressive secretome (involving NF-kB and TGFβ signaling) that alters macrophage function and ultimately promotes cytotoxic CD8T cell dysfunction. Hence, we uncovered a new role of MDK in generating immunologically infiltrated, but otherwise tolerant backgrounds. From a therapeutic perspective, we found that inhibiting MDK restores sensitivity to immune checkpoint blockers in clinical trials. Furthermore, we found an MDK-associated gene set associated with resistance to immunotherapy in several cohorts of patients (Figure 1). Our data, therefore, has important implications at two levels: first, in our understanding of how melanomas can rewire the immune system, and secondly, provides the proof of principle for MDK inhibition as a strategy to improve the efficacy of immune therapies in clinical testing. Importantly, our findings described here for MDK are not restricted to melanoma. We also found immunomodulatory features of MDK in datasets from lung squamous cell carcinoma, glioma, and kidney renal clear cell carcinoma, thus broadening future applications of this protein in the oncology field.

Reference
- Alitalo, A. & Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene doi:10.1038/onc.2011.602
- Olmeda, D. et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546, 676–680 (2017).
- Cerezo-Wallis, D. et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med. (2020). doi:10.1038/s41591-020-1073-3






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
A very important contribution on cancer research. Congrats, and thanks for sharing.