Our labs expertise lies in the in-depth analysis of metabolic pathway changes by using different mass spectrometric approaches and stable-isotope tracing techniques. We are especially interested in understanding the metabolic rewiring that supports the excessive proliferation, enhanced migration and anti-oxidant defense of cancer cells. Our hope is, that the identification of cancer cell specific metabolic vulnerabilities offers the opportunity to selectively target dependencies of tumour tissue in contrast to normal tissue.
Metastatic progression from a primary solid tumour is associated with a dismal prognosis for cancer patients and accounts for a majority of cancer-related deaths. However, most cancer therapies are directed towards cancer growth-inhibition by limiting the biosynthesis of proteins, lipids and nucleotides and do not necessarily interfere with metastatic progression itself. This is why in our current work1, we aimed to identify metabolic pathways that support cancer cell migration and metastasis independent of cell growth.
We find that nucleotide synthesis inhibition and proliferation arrest by antifolates still permits cancer cell migration and show that such migration can be reduced by combinatory metabolic targeting of serine catabolism within the mitochondrial one-carbon cycle.
Nucleotide Synthesis Inhibition and Subsequent Growth Arrest do not Reduce Cell Migration
We employed a panel of different established drugs that are known to target essential metabolic pathways such as glycolysis, electron transport chain or lipid, protein, and nucleotide synthesis. As expected, all perturbations inhibited cancer cell proliferation. But, to our great surprise and in contrast to other growth-inhibiting conditions, inhibition of nucleotide synthesis did not diminish cell migration (Figure 1) in correlation with cell growth reduction.

Figure 1: Correlation of Cell Migration and Cell Growth in Response to Diverse Metabolic Perturbations (SIR = sirolimus, SIM = simvastatin, Rot = rotenone, Gal = galactose, MTX = methotrexate, CLO = clofarabine, PEM = pemetrexed, HU = hydroxyurea). Cell migration was quantified as area under curve of wound closure over 40 h in wound healing assay. Cell growth was quantified as fold change in cell number after 48 h treatment.
This indicated to us that nucleotide synthesis inhibition is in contrast to other metabolic perturbations not effective to also hinder cancer cell migration and prompted us to investigate which metabolic pathways are facilitating this migratory phenotype.
Using methotrexate (MTX) as a tool compound, we found to our surprise that central carbon metabolism and energy status in such growth-arrested cells was not strikingly affected. In fact, glucose and glutamine uptake and catabolism through glycolysis and TCA cycle were not significantly affected by nucleotide synthesis inhibition under MTX.
Cell Migration upon Antifolates is facilitated by Serine Catabolism in Mitochondrial One-Carbon Cycle
Mechanistically, the antifolate MTX blocks regeneration of tetrahydrofolate (THF) by inhibiting dehydrofolate reductase (DHFR). This lack of THF is supposed to fully abrogate one-carbon metabolism, as free THF is needed as a cofactor for enzymatic conversion of serine to glycine (Figure 2). However, we found that cells were still able to release formate under MTX treatment and, using a fully labelled 13C-serine tracer, were able to demonstrate that the released formate was derived from the mitochondrial part of one-carbon metabolism.

Figure 2: One-Carbon Metabolism Catabolizes Serine for Purine- and Pyrimidine-Synthesis. PHGDH, Posphoglycerate Dehydrogenase; SHMT1/2, Serine Hydroxymethyltransferase 1/2; MTHFD1/2, Methylenetetrahydrofolate Dehydrogenase 1/2; TS, Thymidylate synthase; DHFR, Dihydrofolate Reductase; dTTP, Deoxythymidine Triphosphate; For, Formate; MTHFD1L, Methylenetetrahydrofolate Dehydrogenase; THF, Tetrahydrofolate; DHF, Dihydrofiolate; Ser, Serine; Gly, Glycine; 5,10-CH2-THF, 5,10-Methylenetetrahydrofolate; 10-CHO-THF, 10-Formyltetrahydrofolate.
Thereby, we could show that mitochondria preserve THF under antifolate treatment and continue to catabolize serine to formate in an autarkic mitochondrial one-carbon metabolism. Having previously demonstrated the relevance of the one-carbon cycle for cell invasion2, the next logical question to us was therefore: “If we target the observed mitochondrial serine catabolism, can we thereby reduce the observed migratory phenotype under MTX?”
And indeed, we could show that either limitation of serine de novo synthesis or direct genetic targeting of mitochondrial one-carbon cycle are effective to reduce the observed migratory phenotype in vitro under MTX. Finally, we were also able to confirm that mitochondrial one-carbon metabolism is essential for metastatic progression of orthotopic breast tumors in vivo.
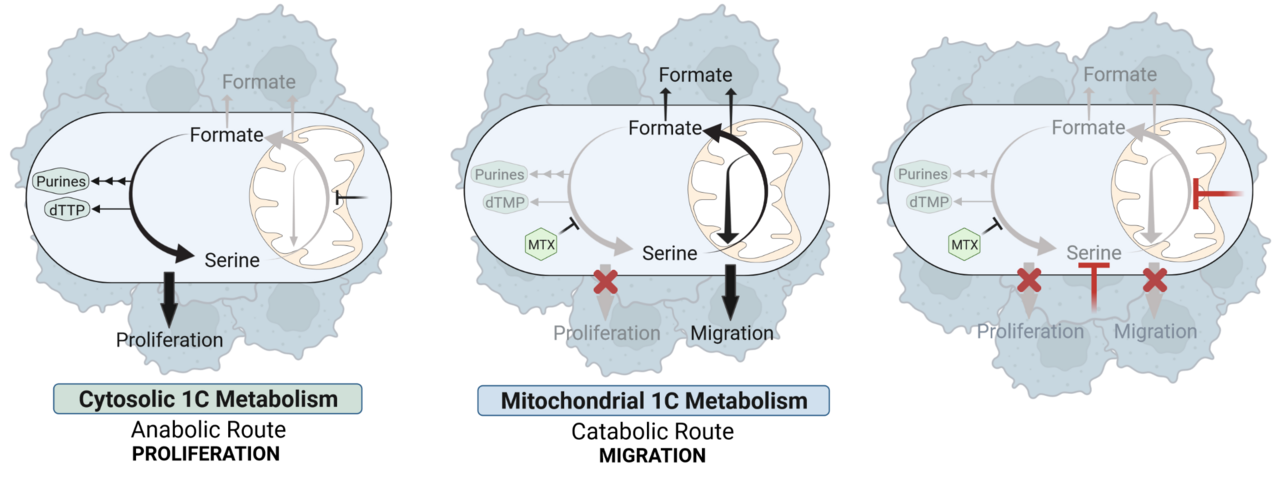
Based on our findings we conclude a model in which cytosolic and mitochondrial one-carbon mechanism can function as autarkic metabolic pathways that have independent relevance for either cell proliferation or cell migration (Figure 3). While the cytosolic one-carbon cycle is mainly important for cell proliferation and can be targeted by antifolates, the mitochondrial one-carbon cycle remains unaffected upon these drugs and sustains cancer cell migration.

Figure 3: Targeting of Cytosolic and Mitochondrial One-Carbon Metabolism Reduces Cancer Cell Proliferation and Migration.
Of note, our work is complemented by a parallel publication from the labs of our colleagues Gerta Hoxhaj, Issam Ben-Sahra and Brendan Manning who show enhanced serine de novo synthesis rates and increased cell migration in melanoma cells in response to purine depletion under MTX3.
Outlook and Beyond
In our opinion, the obtained results within this project once more demonstrate that metabolic pathway evaluation goes beyond the assessment of a pathways biosynthetic capacity and the simple question “Which substrates are consumed and which products are generated?” Instead, it seems important to also consider the two other pillars of cell metabolism: energy dynamics and redox control (Figure 4)4. For example, while MTX treatment unarguably prevents biosynthesis of nucleotides and thereby arrests cell proliferation, unaltered catabolism of serine in the mitochondrion under MTX equips the cells with energy from the ETC and balances redox capacity in addition to allowing synthesis of formate. As a result, all these metabolic outputs from mitochondrial one-carbon metabolism can be used to support cell migration. In light of this, the relative importance of each of these pillars for mitochondrial one-carbon metabolism associated cell migration will certainly be a focus of future research in our lab. 
Figure 4: Three Pillars of Cell Metabolism. Metabolic pathway changes most likely not only affect biosynthesis, but also impact energy dynamics and redox balance within a cancer cell.
Furthermore, we are particularly interested in translating our current findings into further in vivo studies. This will seek to improve the understanding of cancer cell migration facilitated by mitochondrial serine catabolism in growth-arrested cancer cells under chemotherapy, thereby solidifying the translational and therapeutic relevance of the work.
About us
The Luxembourg Institute of Health (LIH) is a public biomedical research organization focused on precision health and invested in becoming a leading reference in Europe for the translation of scientific excellence into meaningful benefits for patients. The research focus of the Cancer Metabolism Group (CMG) at LIH is herein set on the aim to understand the anabolic and catabolic demands of cancer cells in respect to their specific microenvironment to identify key determinants that unbalance metabolic homeostasis leading to disease.
References
1 Kiweler, N. et al. Mitochondria preserve an autarkic one-carbon cycle to confer growth-independent cancer cell migration and metastasis. Nature Communications 13, 2699, doi:10.1038/s41467-022-30363-y (2022).
2 Meiser, J. et al. Increased formate overflow is a hallmark of oxidative cancer. Nature Communications 9, 1368, doi:10.1038/s41467-018-03777-w (2018).
3 MSoflaee, M.H., Kesavan, R., Sahu, U. et al. Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway. Nature Communications 13, 2698, doi:10.1038/s41467-022-30362-z (2022).
4 Benzarti, M., Delbrouck, C., Neises, L., Kiweler, N. & Meiser, J. Metabolic Potential of Cancer Cells in Context of the Metastatic Cascade. Cells 9, doi:10.3390/cells9092035 (2020).
Images were prepared with BioRender.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in