What is the role of AR in CRPC progression?
Castration-resistant prostate cancer (CRPC) is an aggressive lethal form of prostate cancer (PCa) with high metastatic potential and a major therapeutic challenge. The main driver of CRPC progression is the androgen receptor (AR), which acts as a transcription factor to suppress or activate many genes involved in cellular proliferation and differentiation. To block the AR and thereby inhibit the progression of PCa, patients are treated in addition to androgen deprivation therapy (ADT) with AR antagonists. However, the treatment is effective only for a short period and eventually leads to drug resistance.
What is the role of AA in the inhibition of CRPC?
The natural compound, atraric acid (AA), was previously identified and characterized as an AR-antagonist. AA inhibits transactivation besides the wild type AR also those AR-mutants that confer therapy resistance to AR-antagonists indicate a different mode of AR-antagonism. Further, AA inhibits growth and induces cancer cell senescence of both castration-sensitive PCa (CSPC) and CRPC cells in monolayers, 3D tumor spheroids. Also, in patient-derived PCa organoids AA treatment induces cellular senescence ex vivo.
What is the specific role of AA in the inhibition of angiogenesis?
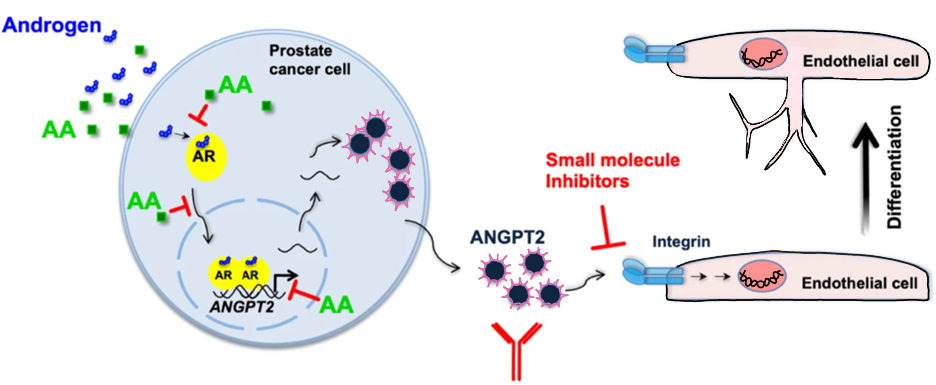
Interestingly, using a mouse xenograft model for CRPC indicates that AA reduces the number of blood vessels in CRPC cells in vivo. However, the underlying molecular mechanism was unclear. In this study, the C4-2 cells are used as a model system for CRPC to analyze the inhibitory role of AA, both mechanistically and functionally on the androgen-induced angiogenesis.
Transcriptome analysis and qRT-PCR results of xenograft tumours indicate that besides induction of senescence pathways. Natably, among those well-known key angiogenic factors, the pro-angiogenic factor angiopoietin 2 (ANGPT2) is robustly induced by treatment with androgens. Moreover, this androgen-mediated induction is counteracted by AA treatment. Interestingly, analysis of angiogenesis protein arrays revealed that the androgen-induced secretion of ANGPT2 is inhibited by AA treatment in C4-2 cells. Interestingly, treating both HUVECs monolayer and 3D HUVEC spheroids with the secretome of AR-ligand pre-treated C4-2 cells show that androgens functionally promote angiogenesis through secretion of ANPGT2 and AA counter-regulates this process. This is confirmed by immune-depletion of ANGPT2 in the secretome, by inhibition of the integrin receptors using small molecule inhibitors, or by treatment with a conditioned medium from AA-treated C4-2 cells, which all block androgen-induced sprouting of primary human umbilical vein endothelial cells (HUVECs). In accordance, the expression of two main ANGPT2 transcript variants are positively associated with the Gleason score (GS), a grade for PCa aggressiveness. In line with these findings, ANGPT2 expression is inhibited in native patient-derived PCa tumour samples treated ex vivo by AA.
What is the take-home message?
In this study, we provide evidence for a novel mechanism of inhibition of CRPC angiogenesis by AA through ANGPT2/integrin axes is identified for the first time. Our results suggest targeting ANGPT2/integrin axes by AA might be a promising strategy to inhibit CRPC angiogenesis (Figure 1).
Outlook and beyond
It is possible that AA might affect the tumour growth by affecting not only the cancer cells and ECs but also supporting cells that mediate vascular stabilization, for instance, pericytes, smooth muscle cells, stromal cells, immune cells, and other cell types in the tumour microenvironment. Therefore, an in vivo model will be beneficial for this purpose.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in