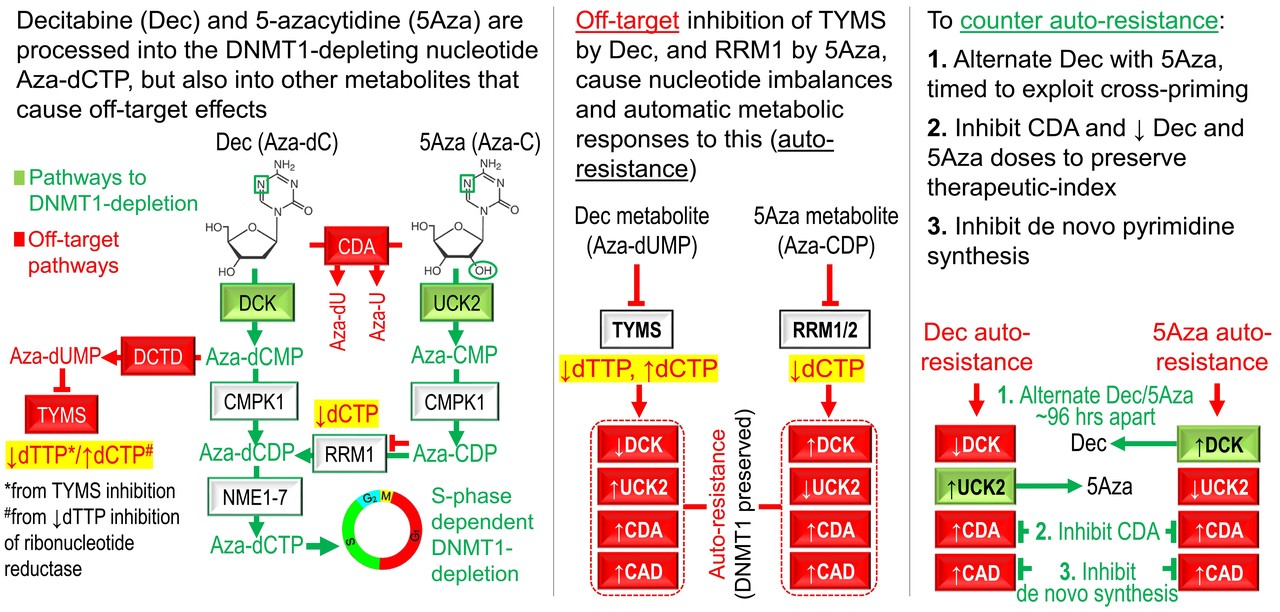
The nub of the matter in oncotherapy is therapeutic index: the need to terminate malignant, but somehow not its close-cousin normal, self-replication. A famous solution is to drug proteins unique to malignant cells, the index example being imatinib to inhibit BCR-ABL in chronic phase chronic myeloid leukemia. Few cancers, however, offer such oncoprotein/drug pairs, and in fewer is the interdicted oncoprotein linchpin enough for durable response. The dream is to have universal targets - to merrily engage genetics be damned - confident that bad but not good will be terminated. Breaking news! We have such targets! But their validation has been gradual and decades-long, by accident/serendipity more than brilliance, obscuring notice by a scientific-industrial complex tuned for dramatically novel story-lines. These targets are corepressors, e.g., DNA methyltransferase 1 (DNMT1), proteins that mediate gene repression as opposed to activation, and recruited in unbalanced fashion into lineage master transcription factor hubs in self-replicating malignant cells. The science behind this is summarized in the Supplementary Discussion of this publication [1] and has been reviewed in detail [2]. The value of these targets is especially apparent when treating myeloid malignancies in the elderly: suppressing malignant clones is only half the battle, the other half is that functional hematopoiesis, already diminished by age and disease processes, must recover - it is no coincidence that DNMT1-depleting drugs decitabine and 5-azacytidine are the only agents approved to treat all genetic/histological sub-types of myelodysplastic syndromes (MDS), and are also backbone drugs to treat acute myeloid leukemia (AML). But clinical responses occur in only ~40% of MDS/AML patients, and relapses are routine. Wait a minute, didn’t we just assert genetics agnostic target validity? Wherefore, therefore, refractory disease/relapse? We wondered the same thing, after observing relapses following initial gratifying responses, to decitabine administered by doses and schedules carefully rationalized to engage DNMT1 without off-target cytotoxicity [3, 4]. We found that MDS/AML resistance was not by selection for genetically mutated sub-clones, but organic/physiologic, an automatic, adaptive response of the pyrimidine metabolism network to drug-induced nucleotide perturbations. Metabolic configurations in histologies other than myeloid are naturally less conducive to decitabine/5-azacytidine processing into DNMT1-depleting nucleotide, suggesting a need for counter-measures, as described [1], from the get go. These discoveries suggest concrete practical steps to widen and extend responses, but also reinforce DNMT1 target-validity – resistance is by evading DNMT1 target-engagement. The practice of oncology requires humility. This can shade to nihilism - “Nobody knows how decitabine or 5-azacytidine or Drug X really works” - bred of the complexity of pharmacology let alone biology, routine failure, dogma and the separate siloes clinicians, scientists, and pharma executives inhabit. Convention, abetted by this strain, excuses more-is-better, empiric, toxic (but often futile) application of oncotherapeutics. Thus, our pick of the take-aways is this: DNMT1 and other specific corepressors (e.g., SMARCA5, CHD4), aberrantly enriched in lineage master transcription factor hubs in self-replicating malignant cells, are the Holy Grail (Excellent Therapeutic Index) targets for therapy we have all been waiting for, let’s engage😊
1. Gu X, Tohme R, Tomlinson B, Sakre N, Hasipek M, Durkin L, et al. Decitabine- and 5-azacytidine resistance emerges from adaptiveresponses of the pyrimidine metabolism network. Leukemia in press.
2. Velcheti V, Schrump D, Saunthararajah Y. Ultimate Precision: Targeting Cancer but Not Normal Self-replication. American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting 2018 May 23; (38): 950-963.
3. Saunthararajah Y, Sekeres M, Advani A, Mahfouz R, Durkin L, Radivoyevitch T, et al. Evaluation of noncytotoxic DNMT1-depleting therapy in patients with myelodysplastic syndromes. J Clin Invest 2015 Mar 2; 125(3): 1043-1055.
4. Awada H, Mahfouz RZ, Kishtagari A, Kuzmanovic T, Durrani J, Kerr CM, et al. Extended experience with a non-cytotoxic DNMT1-targeting regimen of decitabine to treat myeloid malignancies. Br J Haematol 2019 Nov 17.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in