Kras/Lkb1 mutant NSCLC are resistant to standard chemo-immunotherapy
The KL (Kras/Lkb1) subtype of non-small cell lung cancer (NSCLC), where co-mutation of Kras and Lkb1/Stk11 occurs, poses a clinical challenge due to the lack of responsiveness to all the current therapies available today. Approximately half of NSCLC patients with activating Kras mutations have deletions or inactivating mutations in the serine/threonine kinase 11 gene, Stk11 (Lkb1)1,2 which are associated with poor prognosis due to an increase in metastatic burden and resistance to therapy 1,3,4. Cytotoxic chemotherapy was the standard of care for the majority of patients diagnosed with metastatic NSCLC, irrespective of the histological subtype until the recent approval of immune checkpoint blockade therapy (ICB)5. Currently, chemotherapy plus ICB is approved for resectable NSCLC as neoadjuvant therapy and as first-line therapy for metastatic NSCLC. For metastatic NSCLC, carboplatin plus pemetrexed combined with pembrolizumab improved overall survival (OS) relative to chemotherapy alone6,7. These improvements were observed among patients with higher programmed death protein ligand 1 (PD-L1) expression and with no oncogenic driver mutations such as EGFR, ALK, or ROS1. Worse OS and progression-free survival PFS (PFS) outcomes were found in patients with KL subtypes receiving chemotherapy and immunotherapy alone, or in combination, as compared with their wild type groups 4.
Pathogenesis of immune resistance in KL subtypes of NSCLC
The activation of Kras in cancer cells alters the surrounding tumor microenvironment, which is associated with higher PD-L1 expression in tumors through hyper-activation of ERK1/2 signaling and is often responsive to immunotherapy8. Loss of Stk11/Lkb1 gene in tumors confers resistance to ICB as monotherapy or in combination in advanced NSCLC1,4. The co-occurring mutation/deletion of Lkb1 with Kras displays a unique immune contexture in tumors, which is considered as a “cold-tumor” and associated with immune resistance due to the presence of low or no PD-L1, low T-cell densities, high levels of granulocyte colony stimulating factor and IL-8 family cytokines, high density of immune suppressive myeloid cells such as TAM and TAN cells, and production of myeloid cell-recruiting chemokines such as IL-6 9-12. More recently, silencing of STING signaling was found to be associated with immune evasion in KL cells, which might impair innate activation and antigen presentation 13.
TUSC2 immunogene therapy modulates tumor microenvironment and alters the outcome of chemo-immunotherapy in KL subtypes of NSCLC
TUSC2, a tumor suppression gene, is completely absent or weakly expressed in the majority of NSCLCs. Our group has been working on TUSC2 gene therapy for over a decade and established TUSC2 as a potent multi-kinase inhibitor, which induces apoptosis in cancer cells. The safety of nanovesicle mediated-TUSC2 delivery was shown in a phase I study 14 and currently, a phase I/II clinical trial evaluating TUSC2 synergy with a third generation TKI, osimertinib, is recruiting NSCLC patients (ClinicalTrials.gov Identifier: NCT04486833). We have recently identified TUSC2 as an immunogene due to its notable role in selectively augmenting the natural killer (NK) cells and cytotoxic T lymphocyte (CTL) activity in the tumor microenvironment15. TUSC2 also downregulates PD-1/PD-L1 signaling and synergizes with anti-PD-1 in syngeneic Kras mutant lung cancer mouse models15,16. In this article, the authors tested the hypothesis that TUSC2 immunogene therapy would modulate the immune-cold KL-mutant tumor microenvironment through promoting a variety of innate and adaptive immune responses and would alter the outcome of chemo-immunotherapy combination in a humanized mouse model. The synergistic antitumor effect was associated with increased activation of antigen-presenting cells for antigen processing and presentation, CTL responses, and increased accumulation of antigen-specific memory T cells in tumors.
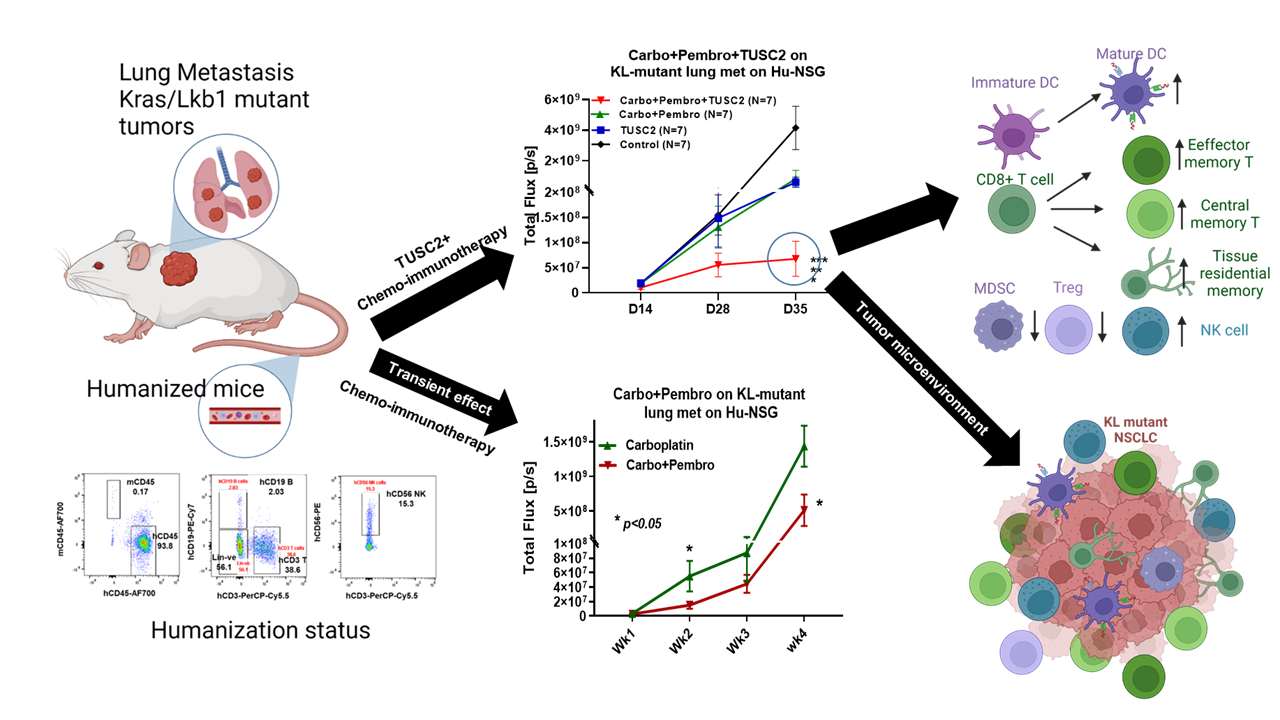
Experimental approach: Highly advanced humanized mouse model used for preclinical activity
To test the antitumor activity of TUSC2 immunogene therapy and its combinations with immunotherapy or chemo-immunotherapy in preclinical models, we have used a humanized mouse model that recapitulates the broad-spectrum human immune system in mice. Our group recently developed an improved humanized mouse model using fresh human umbilical cord blood derived CD34+ stem cells 17. The reconstituted humanized mice have a fully competent human immune system with major functional immune populations, which showed antigen specific T cell responses as well as antitumor activity with immune checkpoint blockade therapy. This model provides a unique opportunity for establishing an effective drug screening workflow for immunotherapy. The experimental lung metastasis from KL mutant A549 NSCLC was developed in humanized mice and the chemo-immunotherapy (carboplatin + pembrolizumab) showed an additive antitumor response which is non-durable, transient and similar to the response reported in KL NSCLC patients19, 22. TUSC2 immunogene therapy showed a synergistic antitumor activity in controlling lung metastasis with either pembrolizumab or nivolumab through activation of natural killer (NK) cells and enhancement of CTL responses in the tumor microenvironment. The antitumor activity was many fold greater when TUSC2 was combined with chemo-immunotherapy (carboplatin + pembrolizumab) which eradicated metastases in over 65% of humanized mice implanted with KL NSCLC metastatic tumors. This impressive therapeutic benefit of carbo+pembro+TUSC2 was reproduced in three blinded independent experiments, where the triple treatments showed the best antitumor effect. The carboplatin+pembrolizumab effect was insignificantly different from TUSC2 alone.
The TUSC2+chemo-immunotherapy combination induced strong antitumor immune responses in humanized mice
The robust antitumor immune responses from the triple combination altered the immune contexture in KL tumors towards a pro-immune tumor microenvironment, which is characterized by significant infiltration of activating CTL and NK cells. Chemo-immune treatment as well as triple treatment induces a significant number of mature antigen presenting HLA-DR+ DC and CD11c+ DC, suggesting the role of chemotherapy in releasing tumor antigens for processing and presentation by APCs. Similar to clinical observations, pembrolizumab was able to block PD-1 expression completely on TILs in humanized mice. The addition of TUSC2 with chemo-immunotherapy altered immunosuppressive component in the tumor microenvironment by downregulating Treg, MDSC and M2 macrophages significantly better than other combinations tested. The complete eradication or significant reduction of KL tumor nodules in the lungs by the triple treatment was associated with an antigen-specific T cell response primarily consisting of central memory (CM), effector memory (EM) and CD103+ residential memory (TRM) T cells (Figure 1). Influx of IFN-g+ CM or EM CD8+ T cells was found in tumors treated with TUSC2+chemo-immunotherapy and depletion of these CD8+ T memory cells drastically reduced the functionally active IFN-g producing T cells in tumors. To the best of our knowledge, this is the first study with an in depth analysis of human memory T cells in humanized mice.
Future prospect
This study showed that TUSC2 immunogene therapy synergizes with standard chemo-immune combination therapy and overcomes resistance to checkpoint blockade in the KL subtype of NSCLC supporting the addition of immunogene therapy to standard combinations in future clinical trials.
1 Skoulidis, F. et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer discovery 8, 822-835, doi:10.1158/2159-8290.CD-18-0099 (2018).
2 Facchinetti, F. et al. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer 112, 62-68, doi:10.1016/j.lungcan.2017.08.002 (2017).
3 Basher, F., Saravia, D., Fanfan, D., Cotta, J. A. & Lopes, G. Impact of STK11 and KRAS co-mutations on outcomes with immunotherapy in non-small cell lung cancer. J Clin Oncol 38 (2020).
4 Shire, N. J. et al. STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PloS one 15, e0238358, doi:10.1371/journal.pone.0238358 (2020).
5 Schiller, J. H. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. The New England journal of medicine 346, 92-98, doi:10.1056/NEJMoa011954 (2002).
6 Gandhi, L. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 378, 2078-2092, doi:10.1056/NEJMoa1801005 (2018).
7 Jotte, R. et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 15, 1351-1360, doi:10.1016/j.jtho.2020.03.028 (2020).
8 Liu, C. et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett 470, 95-105, doi:10.1016/j.canlet.2019.10.027 (2020).
9 Koyama, S. et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer research 76, 999-1008, doi:10.1158/0008-5472.CAN-15-1439 (2016).
10 Skoulidis, F. et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer discovery 5, 860-877, doi:10.1158/2159-8290.CD-14-1236 (2015).
11 Biton, J. et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 5710-5723, doi:10.1158/1078-0432.CCR-18-0163 (2018).
12 Bange, E. et al. Impact of KRAS and TP53 Co-Mutations on Outcomes After First-Line Systemic Therapy Among Patients With STK11-Mutated Advanced Non-Small-Cell Lung Cancer. JCO Precis Oncol 3, doi:10.1200/PO.18.00326 (2019).
13 Kitajima, S. et al. Suppression of STING Associated with LKB1 Loss in KRAS-Driven Lung Cancer. Cancer discovery 9, 34-45, doi:10.1158/2159-8290.CD-18-0689 (2019).
14 Lu, C. et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PloS one 7, e34833, doi:10.1371/journal.pone.0034833 (2012).
15 Meraz, I. M. et al. TUSC2 Immunogene Therapy Synergizes with Anti-PD-1 through Enhanced Proliferation and Infiltration of Natural Killer Cells in Syngeneic Kras-Mutant Mouse Lung Cancer Models. Cancer immunology research 6, 163-177, doi:10.1158/2326-6066.CIR-17-0273 (2018).
16 Cao, X. et al. TUSC2 downregulates PD-L1 expression in non-small cell lung cancer (NSCLC). Oncotarget 8, 107621-107629, doi:10.18632/oncotarget.22581 (2017).
17 Meraz, I. M. et al. An Improved Patient-Derived Xenograft Humanized Mouse Model for Evaluation of Lung Cancer Immune Responses. Cancer immunology research 7, 1267-1279, doi:10.1158/2326-6066.CIR-18-0874 (2019).






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in