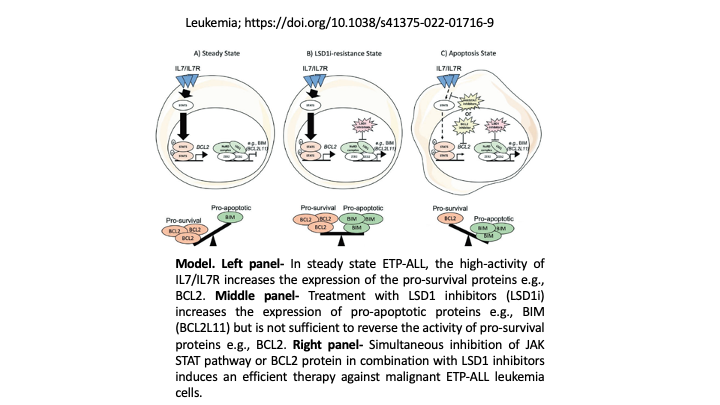
The Haigh lab at the CancerCare Manitoba Research Institute (CCMR), has previously demonstrated that increased expression of the ZEB2 transcription factor, that binds to DNA and controls key genetic programs, can specifically transform murine T cells during an early stage in their development (Goossens et al., Nature Communications 2015). This leads to defects that resemble the disease observed in early thymic progenitor acute lymphoblastic leukemia (ETP-ALL) patients. ETP-ALL can occur in both children and adults and is quite aggressive and difficult to cure and often relapses following therapy. In both mouse and human ETP-ALL, the IL7-R-JAK/STAT pathway is known to play an important role in increasing pro-survival factors such as BCL2 as well as interfering with normal T cell development (Goossens et al., Haematologica 2019). Using a proteomics-based approach the Haigh lab found that ZEB2 can interact with epigenetic factors such as KDM1A (LSD1) and alters its activity (Goossens et al., Blood Journal 2017). As a result, LSD1 inhibition (LSD1i) has been proposed as a way of differentiating and killing leukemic cells with high ZEB2 levels. However, the Haigh lab has now found that the IL7-R pathway and BCL2 play a major role in resistance to LSD1i, particularly in human ETP-ALL cells. Using a bioinformatics and mRNA expression profiling-based approach Benyoucef et al., have now discovered that ZEB2 in complex with LSD1 can repress many pro-death or pro-apoptotic genes including BCL2L11 (BIM). They have demonstrated using both primary mouse and human ETP-ALL patient cells that in order to effectively kill these cells one must use a allosteric LSD1 inhibitor (SP2509) that preferably breaks up the repressive complex formed between ZEB2 and LSD1 that allows restored BIM expression. Combining SP2509 with either IL7-R signalling blockade using inhibitors against downstream JAK2 (Ruxolitinib) or inhibitors that directly interfere with the pro-survival BCL2 protein (ABT-199 or Venetoclax) effectively results in ETP-ALL cell differentiation and shifts the survival factor balance towards BIM-induced apoptosis. This novel combination therapy holds great promise for treating aggressive forms of leukemia such as ETP-ALL.
ZEB2 controls the balance of Apoptosis Factors in ETP-ALL.
Cell survival is based upon a tightly regulated balance between pro-survival and pro-apoptosis inducing factors. Pro-survival factors can drive chemotherapy resistance in leukemia. Overcoming resistance, cells must undergo a shift in favour of pro-apoptosis signals to effectively kill leukemia.
Published in
Cancer

Subscribe to the Topic
Cancer Biology
Life Sciences > Biological Sciences > Cancer Biology
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in